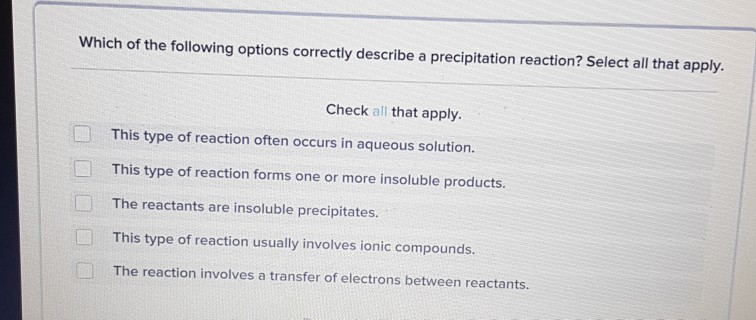
Which of the Following Options Correctly Describe a Precipitation Reaction
Categorize the following reaction as an acid-base neutralization precipitation combination decomposition combustion displacement or disproportionation reaction. When Ag reacts with C l we get a white precipitate of AgC l that is silver chloride.

Che 140 Ch 6 Learn Smart Flashcards Practice Test Quizlet
Web based tutoring advising center management and tracking software.

. E CuO 2HCl to Cl 2 H 2 O. When Acetic acid reacts with a base let us suppose Calcium Hydroxide that is C aOH2. Which of the following options correctly describes a precipitation reaction.
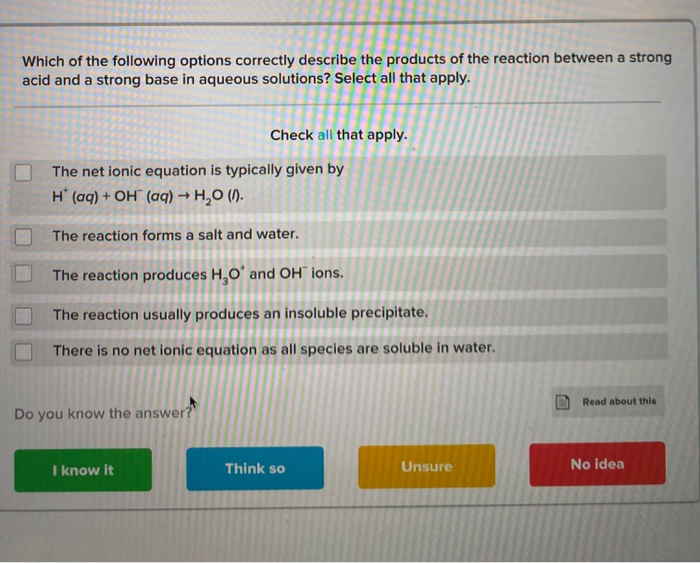
Precipitation reactions are usually double displacement. Select all that apply. Which of the following options correctly describe the products of the reaction between a strong.
One of the products of this reaction would be _____. This is the basis of redox reactions. Ag C l AgC l.
The reactants are insoluble precipitates. Which of the following options correctly describe the analysis of the Fe2 in the water sample. These insoluble salts formed in precipitation reactions are called precipitates.
NiNO 3 2 aq K 2 Saq NiS 2KNO 3 aq. Online tracking and scheduling and multiple learning advising center monitoring. The term precipitation reaction can be defined as a chemical reaction occurring in an aqueous solution where two ionic bonds combine resulting in the formation of an insoluble salt.
D Na 2 SO 4 pbNO 3 to PbSO 4 2NaNO. When an element is oxidized it loses electrons. Which of the following options correctly describe a precipitation reaction.
H C 2 H 3 O2 lOH aq H 2 OOC 2 H 3 O2 aq Option A and D are correct answers. Meanwhile a reducing agent reduces something else and gets oxidized in the process losing. B Fe H 2 SO 4 to FeSO 4 H 2.
Select all that apply. Use the solubility rules to determine which compounds forms precipitates if any. A Write a balanced molecular equation for the reaction between aqueous solutions of acetic acid CH 3 COOH and barium hydroxide BaOH 2.
However an oxidizing agent oxidizes something else and gets reduced therefore gaining electrons. This type of reaction usually involves ionic compound C. The statements that correctly describe chemical reactions are.
A Combination reaction D Disproportionation reaction B Acid-base neutralization reaction E Combustion reaction C Hydrogen displacement reaction Ans. Ag aq NO 3aq K aq Cl aq AgCl s K aq NO 3aq A final way to represent a precipitation reaction is known as the net ionic equation. If the reactants include an aluminum atom then the products must include an aluminum atom.
But when an element is reduced it gains electrons. The vant Hoff factor is predicted from the number of particles the electrolyte will form after dissociation. This type of reaction forms one or more insoluble products E.
3 Agaq PO 4 3aq Ag 3 PO 4 s 4. Which of the following represents a precipitation reaction. The reaction involves a transfer of electrons between reactants D.
Above reaction is a double displacement reaction as ions both cations and anions of the reacting molecules are exchanged. C 4HCl MnO 2 to MnCl 2 2H 2 O Cl 2. The reactants are insoluble precipitates.
Which of the following species should be included in the net ionic equation for the precipitation reaction given. This is known as the complete ionic equation. A H 2 SO 4 NaCl to NaHSO 4 HCl.
Third option is the correct one. The vant Hoff factor is greater than 1 for electrolyte solutions The vant Hoff factor is use to predict the colligative properties of an. This type of reaction often occurs in aqueous solution.
This reaction can be also be written in terms of the individual dissociated ions in the combined solution. If the reactants include 5 atoms of Fe then the products must include 5 atoms of Fe. Water is formed along with Calcium acetate which is a white.
Select all that apply. Precipitation Reaction Definition and Meaning. Which of the following options correctly describe the vant Hoff Factor for the solution of an electrolyte.
There are 26 10-4 moles of Fe2 in the water sample. A precipitation reaction is a reaction in which two or more water- _____ ionic compounds react in aqueous solution to form one or more _____ precipitates. If there are a total of 15 atoms in the reactants there must be a total of 15 atoms in the product.
Barium ion B a 2 combines with sulphate ion S O 4 2 to form Barium sulphate B a S O 4 and ammonium ion N H 4 combines with chloride ion C l ion to form ammonium chloride N H 4 C l. BaC 2 H 3 O. Write the net ionic equation for the precipitation reaction that occurs when aqueous solutions of silver nitrate and potassium phosphate are mixed.
This type of reaction forms one or more insoluble products. 44 A precipitation reaction takes place when aqueous cobaltIII chloride reacts with aqueous lithium hydroxide. Both are precipitation reaction.
A CoOH 3 s B CoOH 2 s C LiCO 3 s D LiCl 3 aq E Cl 3 OH s 45 What is the driving force in the following reaction. States of matter have not been included.
Chapter 4 Chem Flashcards Quizlet
Solved The Following Options Correctly Describe The Products Chegg Com
Remote Sensing Free Full Text Good Practices For Object Based Accuracy Assessment Html
8 2 Precipitation Gravimetry Chemistry Libretexts

Combo With C9 C7 And 3 Others Flashcards Quizlet

It Takes Two Las1 Hepn Endoribonuclease Domains To Cut Rna Correctly Journal Of Biological Chemistry

How To Identify A Precipitation Reaction Chemistry Study Com

Combo With C9 C7 And 3 Others Flashcards Quizlet
Solved Which Of The Following Options Correctly Describe A Chegg Com
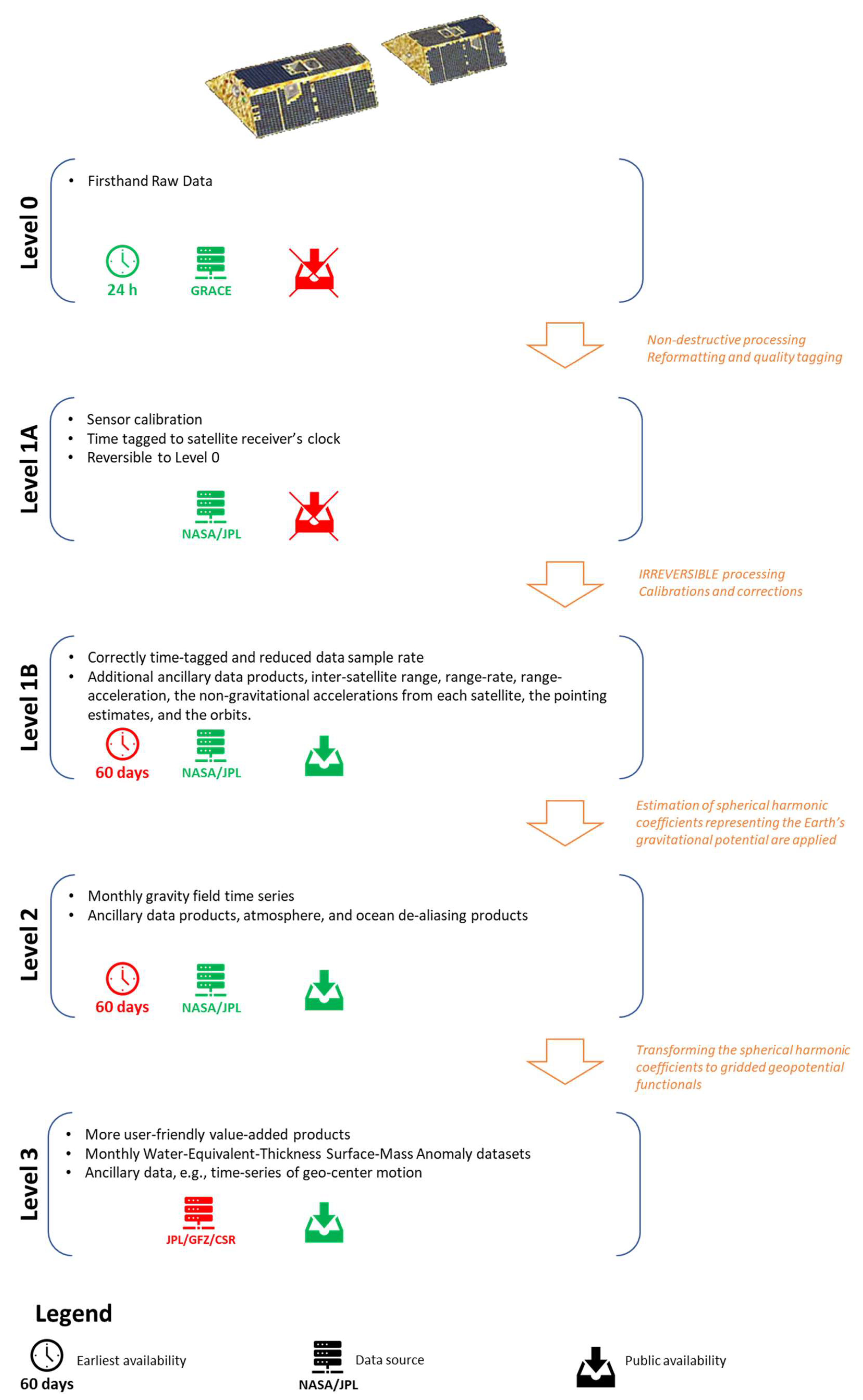
Water Free Full Text An Overview Of Groundwater Monitoring Through Point To Satellite Based Techniques Html

Che 140 Ch 6 Learn Smart Flashcards Practice Test Quizlet

What Do You Mean By A Precipitation Reaction Explain By Giving Examples Chemistry Q A

Che 140 Ch 6 Learn Smart Flashcards Practice Test Quizlet

Combo With C9 C7 And 3 Others Flashcards Quizlet

Quiz Worksheet How To Predict Precipitates And Net Ionic Equations Study Com

Che 140 Ch 6 Learn Smart Flashcards Practice Test Quizlet

Che 140 Ch 6 Learn Smart Flashcards Practice Test Quizlet


Comments
Post a Comment